24++ Derive The Integrated Rate Law For First Order Reaction
Derive The Integrated Rate Law For First Order Reaction. Consider first order reaction, a → b + c. The above equation is known as integrated rate equation for zero order reactions.

1 → 2 → (85) where k1 and k2 are the rate constants for the first and second steps, respectively. T is the time elapsed since the reaction began Because hi is the only reactant and the only species that appears in the rate law, the reaction is also second order overall.
douglas bois prix demi sabot de guidage pour porte de garage coulissante ducati hypermotard 796 deco table violet et rose
Derive Integrated Rate Equation For First Order Reaction
Because hi is the only reactant and the only species that appears in the rate law, the reaction is also second order overall. The differential rate law is given by. ∫ d x ( r − x) = k 1 ∫ d t. The differential rate law is given by.

Ln[ ]=− g p+ln[ ]0 1 → 2 → (85) where k1 and k2 are the rate constants for the first and second steps, respectively. The above equation is known as integrated rate equation for zero order reactions. In this type of reaction, the sum of the powers of concentrations of reactants in rate law is equal to 1, that.

The differential rate law is given by. Ln[ ]=− g p+ln[ ]0 Write an integrated rate law for the reaction that is first order in a and first order in (8) [1] = k[a|| plot a graph that would confirm that the reaction that is first order in a and first order in [b]. The differential rate law is given.

Consider the reaction r→p again. T is the time elapsed since the reaction began The important thing is not necessarily to be able to derive each integrated rate law from calculus, but to know the forms, and which plots will yield straight lines for each reaction order. In this type of reaction, the sum of the powers of concentrations of.

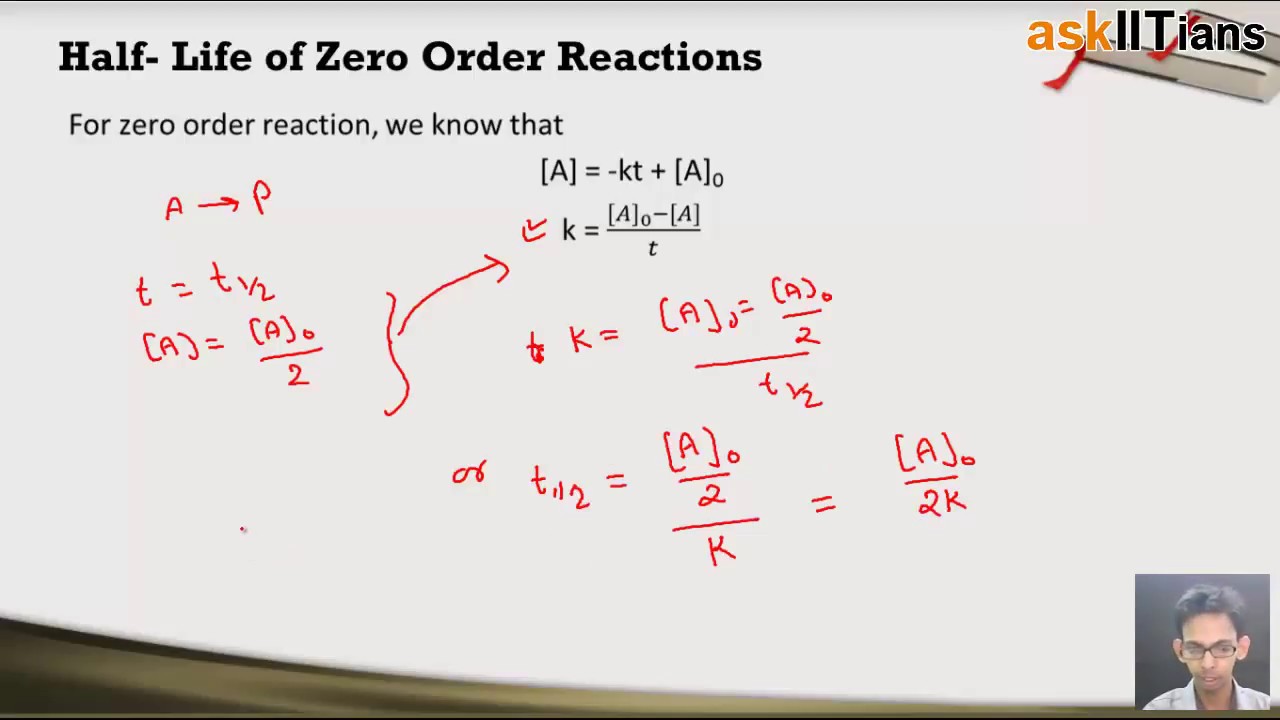
Therefore, the rate law for this reaction is, rate is directly proportional to [r] we know that [r]=−kt+[r] 0. Consider the reaction r→p again. Consider first order reaction, a → b + c. The differential rate law is given by. A summary of the various integrated rate laws, including the different plots that will yield straight lines, can be used.

Consider first order reaction, a → b + c. The equations which are obtained by integrating the differential rate laws and which gives the direct relationship between the concentrations of the reactants and time is called integrated rate laws. The rate of reaction is given by dx/dt. Suppose [a] t is the concentration of a at time = t 1.
Because hi is the only reactant and the only species that appears in the rate law, the reaction is also second order overall. T is the time elapsed since the reaction began Rate law and reaction order (video) | khan academy aug 25, 2020 · b the exponent in the rate law is 2, so the reaction is second order.

Therefore, the rate law for this reaction is, rate is directly proportional to [r] we know that [r]=−kt+[r] 0. T is the time elapsed since the reaction began Consider the reaction r→p again. Rate law and reaction order (video) | khan academy aug 25, 2020 · b the exponent in the rate law is 2, so the reaction is second.